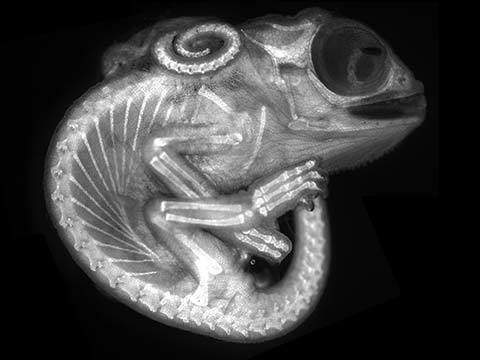
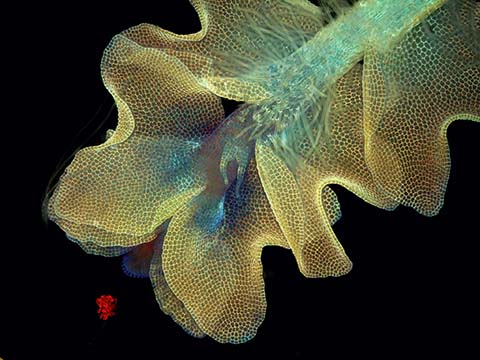
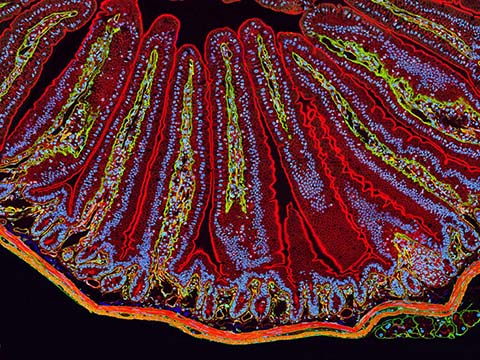
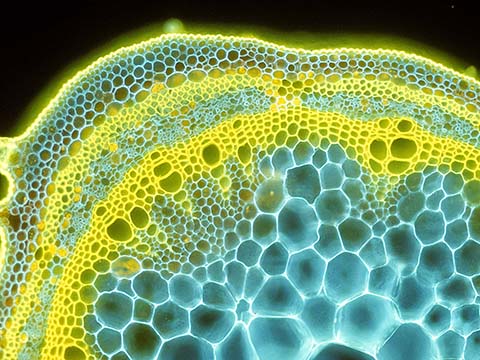
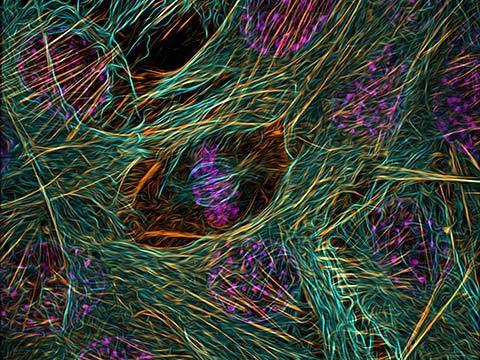
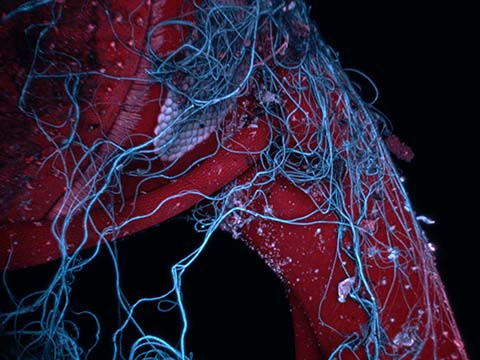
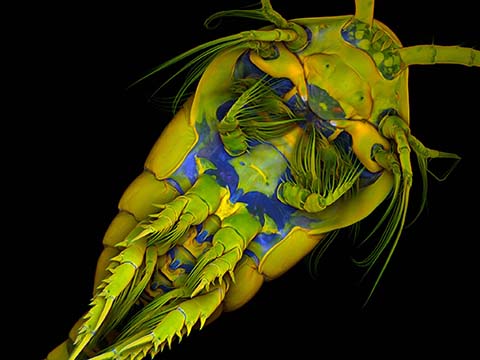
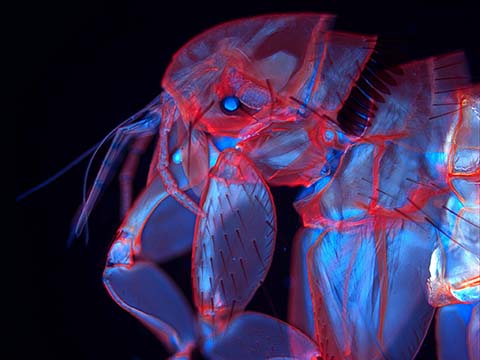
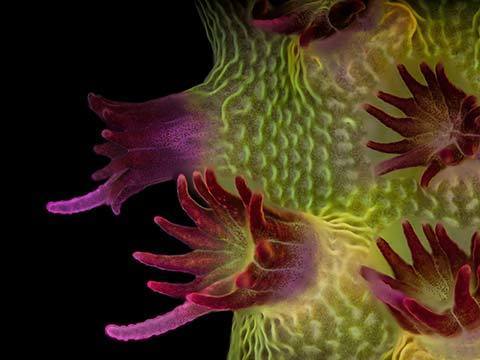
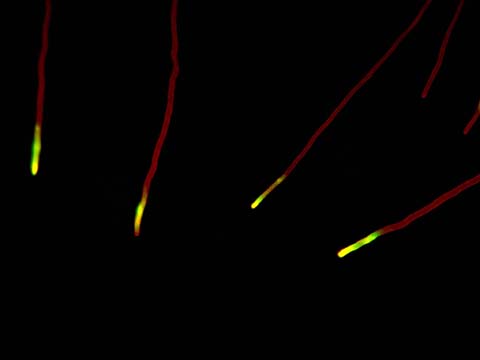
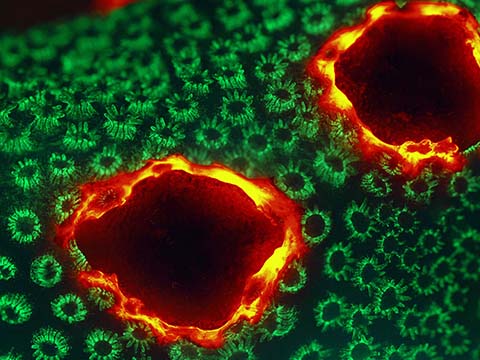
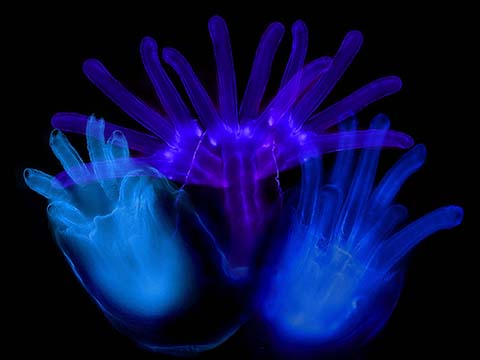
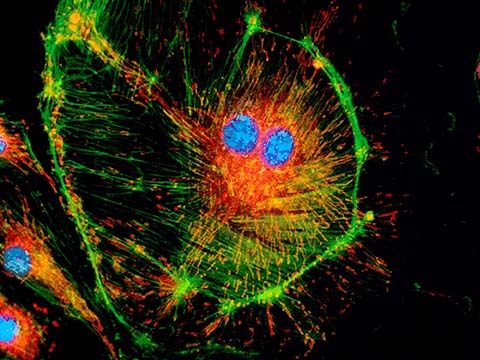
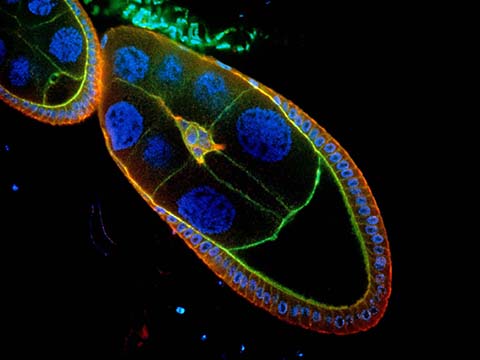
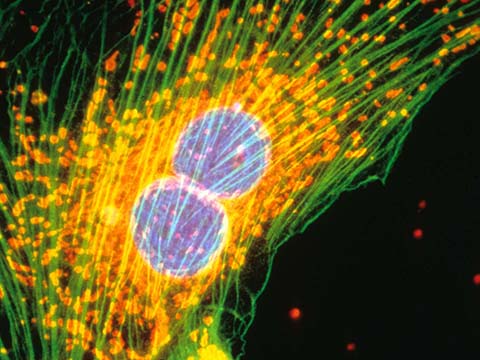
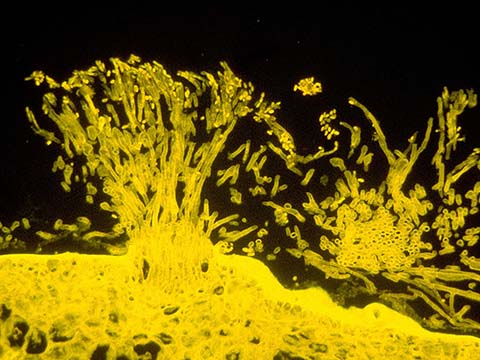
Fluorescence

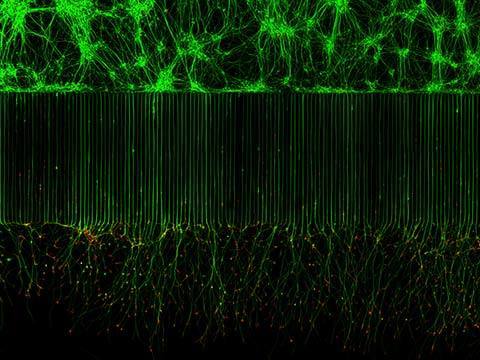
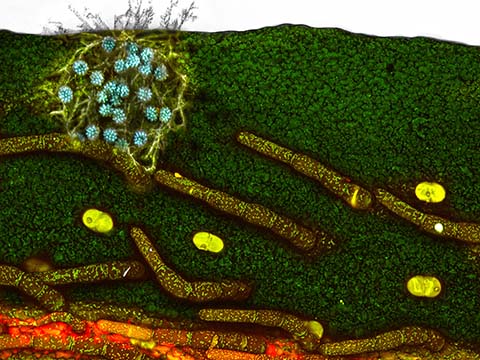
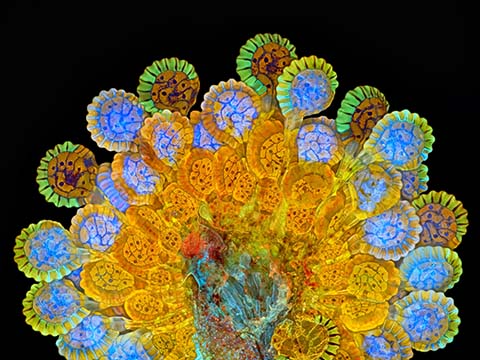
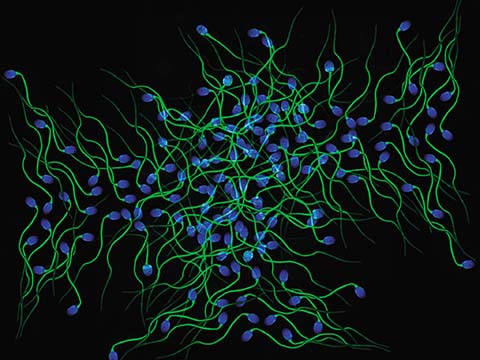
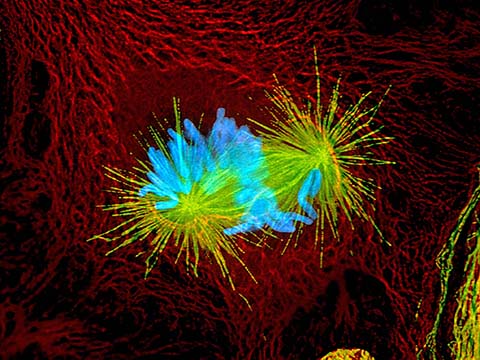
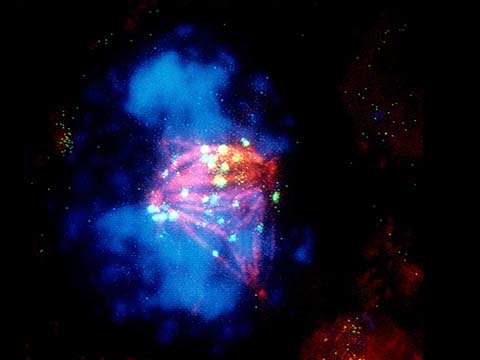
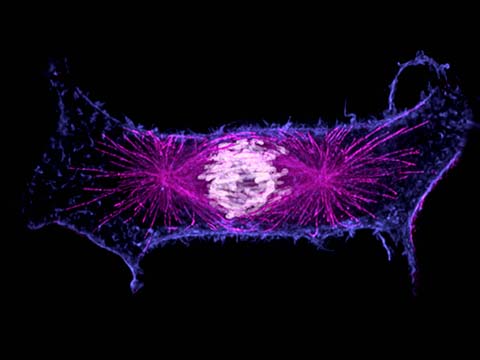
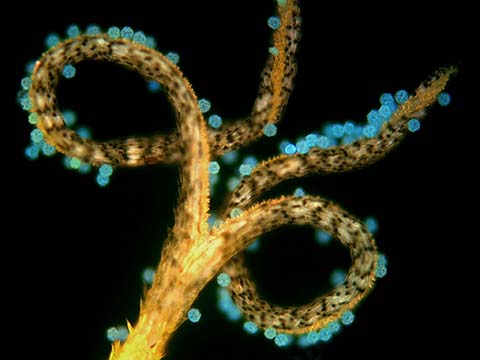
Fluorescence imaging uses high intensity illumination to excite fluorescent molecules in the sample. When a molecule absorbs photons, electrons are excited to a higher energy level. As electrons ‘relax’ back to the ground-state, vibrational energy is lost and, as a result, the emission spectrum is shifted to longer wavelengths.
452 Entries
Displaying 61 – 120
Previous Page Next Page